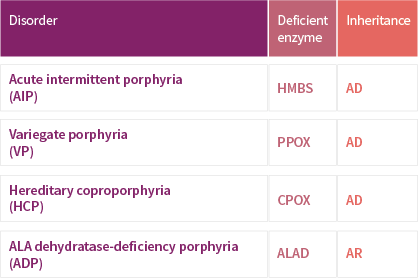
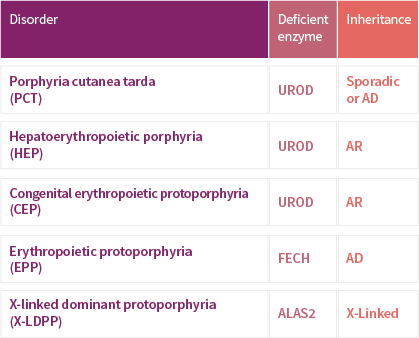
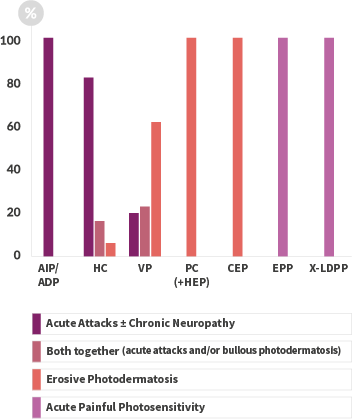
TERMS OF USE
These terms govern your access to the websites controlled by Orphan Europe. These terms do not apply to residents in the United States, or third-party websites to which Orphan Europe websites may link.
Disclaimer
Orphan Europe has developed this website for any person who wishes to find out more about our activities. Although we strive to provide the latest developments relating to our products and services, and other information about Orphan Europe, we do not warrant the accuracy, effectiveness, and suitability of information contained in the Orphan Europe websites. Information provided should not be relied upon in connection with any investment decision. Medical information that appears on this website is for information purposes and is not a replacement for advice given by a physician or other health care professional. Links and references to additional resources and information are provided on the website. Orphan Europe reserves the right to delete or modify information provided on this website at anytime without prior notice.
This website is published as it without any warranty of any kind, express or implied, as to the operation of the website. The accuracy of the information or the products or services referred to on the website (in so far as such warranties may be excluded under any relevant law) and Orphan Europe shall not be liable for any losses or damage that may result from use of the website as a consequence of any inaccuracis in, or omissions from, information which it may contain
Copyright
Copyright in these pages is owned by Orphan Europe. Orphan Europe owns the copyright in the content published on the website except where otherwise indicated by a third party’s proprietary notice. Images, trademarks and brands are also protected by other intellectual property laws and must not be reproduced or appropriated in any manner without written permission of their respective owners. Permission to use the information is only granted for information and non-commercial or personal use only and should not be modified in any way. No use of any Orphan Europe trademark, trade names and products may be made without the prior written authorisation of Orphan Europe, except to identify the product or services of the company.
Cookies
This website may use cookies for measuring website traffic. Orphan Europe may collect information about your visits to this website without you actively submitting such information. This information may include, for example, your browser type and language, your operating system, your IP address, the web search that guided you to this website.
Privacy policy
Orphan Europe collects personal information that you enter into data fields on the Orphan Europe website. For example you may submit your name, email address and a request for additional information on a particular subject. To protect your privacy you should not provide any information that is not specifically requested. Orphan Europe will use the personal information you provide to respond to your question and to provide you with an efficient customer service. The personal information will be accessed by a restricted number of Orphan Europe employees. We train our employees about the importance of privacy and how to handle and manage customer data appropriately and securely. If you have contacted us your e-mail address will be stored on our server but will not be used for commercial purposes. You have a right to access, modify, correct and delete any personal information sent to Orphan Europe, in accordance with the French Data Protection Act of 6 January 1978.
Contact information and policy updates
If you have any question about the use, amendment, or deletion of personal information that you have provided to us, please contact us by clicking on the “contact us” link on the website. Alternatively you may send a letter to the following address:
Orphan Europe SARL
Siret: 379 088 115 00058
Eric Pottier – Chief Pharmaceutical Office
Immeuble « Le Wilson »
70 avenue du General de Gaulle
92800 Puteaux
Tel. + 33 (0) 1 43 73 64 58